Get to know Emily Martin, a graduate student at Iowa State University who is seeking solutions to water problems.
Continue reading2018 Iowa Water Conference (Photo Recap)
Researcher Profile: Elizabeth Swanner
Get to know Elizabeth Swanner, an Assistant Professor in the Department of Geological and Atmospheric Sciences at Iowa State University.
Continue readingOxbows for Conservation and Nutrient Reduction in Iowa Floodplains
Post submitted by Clay L. Pierce, U.S. Geological Survey and Keith E. Schilling, Iowa Geological Survey
Formed when looping stream meanders are cut off through bank erosion or as the result of artificial stream straightening, oxbows are a common geomorphic feature in floodplains of Iowa’s agricultural landscape. Early accounts of habitat use by many fish species in prairie streams in Iowa and other Midwestern states describe habitats such as slow pools, submerged and emergent vegetation, side channels, and backwaters – habitats that are rare or nonexistent in those streams today. Oxbows are frequently among the few remaining slow or standing water habitats associated with many streams in these regions. Previous studies have established oxbows’ conservation value for Topeka shiners, federally listed as an endangered species, as well as their habitat value for numerous other native fish species. Studies conducted over the last two decades in Iowa consistently report greater prevalence and abundances of Topeka shiners in oxbows than in their associated streams. Ten other fish species of greatest conservation need have also been found in Iowa oxbows.
Widespread nutrient loss from agricultural areas in the U.S. Midwest is impacting aquatic ecosystems at both local and regional scales, including the Gulf of Mexico. Enhancing ecosystem services in floodplains offers opportunities to achieve nutrient reduction benefits in agricultural watersheds while minimizing loss of crop production areas. Preliminary studies in Iowa suggest that oxbows intercepting surface and tile water can reduce the amount of nitrate-nitrogen reaching streams by roughly one half.
Ongoing conservation studies, which will be presented in the oxbow track of the upcoming 2018 Iowa Water Conference, describe efforts to identify oxbow remnants for restoration, landscape and habitat characteristics of oxbows associated with presence of Topeka shiners, restoration programs in Iowa to increase the number and quality of oxbows for Topeka shiners, and efforts to evaluate the success of oxbow restorations. Also to be presented in the oxbow track of the 2018 Iowa Water Conference are three studies addressing water quality benefits of oxbows describing quantification of nutrient reduction benefits at individual oxbow sites and exploring the value and benefits of oxbow restorations within a watershed water quality planning context. A comparison of the value of oxbow restorations within the context of statewide nutrient reduction strategies in Iowa will also be discussed.
Clay Pierce is the Assistant Leader of the U.S. Geological Survey’s Iowa Cooperative Fish and Wildlife Research Unit at Iowa State University in Ames.
Keith Schilling is an Associate State Geologist and Research Engineer for the Iowa Geological Survey at the University of Iowa in Iowa City.
Winter Update from the IWC Graduate Student Research Grant Program: Emily Martin
Post submitted by Emily Martin, MS Environmental Science student at Iowa State University and recipient of the Graduate Student Supplemental Research Competition
Since the last update, we switched the focus of our study to the ability of biochar to remove nitrate in comparison to a woodchip-only bioreactor. As a reminder, the original goal of the project was to evaluate the ability of woodchip bioreactors to remove phosphorous by adding biochar as a phosphate (P) amendment. In the previous update, we found in a P sorption study that none of the biochars performed well at removing P from solution.
To compare nitrate removal, we ran what is called a batch reactor test. The batch test used five liter buckets filled with 30 grams of biochar, 350 grams of Ash woodchips, and three liters of deionized water. As a control to see the real impact of adding biochar, some buckets only contained woodchips. Both the test and control buckets had three types of denitrifying microbes added: Bradyrhizobium japonicum, Pseudomonas stutzeri, and DN-8A.
One issue that can arise not only in batch tests, but also in field woodchip bioreactors is an initial flushing of nutrients from the woodchips, and as we found out in the P sorption tests, also from biochar. To prevent this affecting our batch reactor tests, we allowed the mixture to soak for 24 hours. After the initial soak, the buckets were drained of the deionized water and two liters of nutrient solution was added. The nutrient solution was made to 30 mg/L NO3– and 10 mg/L PO42- using KNO3 and KH2PO4 – PO4 with deionized water, respectively. Samples were taken at 0, 4, 8, 12, and 24 hours to test for NO3—N.
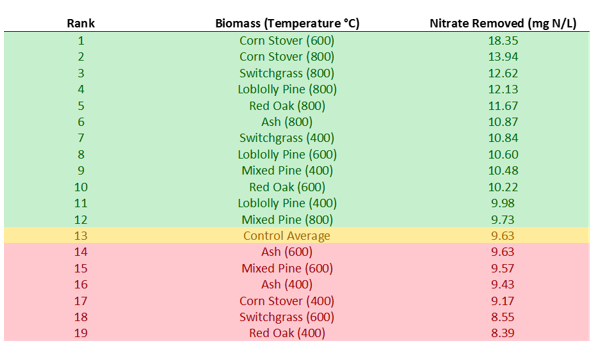
Results showed that 12 of the 18 biochars removed more nitrate than the woodchip control. The biochar with the most removal was the 600°C Corn Stover, which almost doubled the amount of nitrate removed by the control. Of the 12 biochars that removed more nitrate than the control, 50 percent were 800°C, 25 percent were 600°C, and 25 percent were 400°C. All six of the 800°C biochars performed better than the control. The nitrate results overall were more promising than what was found in the P sorption test. There is potential to increase the ability of field bioreactors to remove nitrate by adding biochar; however, more tests will be needed to see how the biochar handles scaling up and field conditions. This was a short-term test in a laboratory setting. It is possible that on a larger scale, longer timescale, and at varying influent nitrate concentrations, biochar could perform worse than seen in the lab.
A secondary part of the batch test was following up to the P sorption test. Because the biochar leached phosphorus in the P sorption test, the 24 hour soak in deionized water should have helped remove the initial leaching. We are still testing all of the biochars, but initial results from a set of three biochars and the woodchip control showed that all still leached phosphorus into the solution. This could be problematic for the use of biochar in field conditions and should be managed if tests are taken to full-scale.
The next step for the project is to finish testing for phosphorus removal from the batch tests. After that, a paper will be written and submitted for publishing. As conferences are coming up this spring, I will be creating a poster to present at the Iowa Water Conference (March 21-22) and the Environmental Science Graduate Student Symposium (April 4).
Research shows social networks play an integral part in conservation practice adoption
Ames, Iowa – Research shows that to meet the goals of the Iowa Nutrient Reduction Strategy, Iowa farmers will need to increase use of a diverse array of appropriate nutrient management and other conservation practices. However, most soil and water conservation practice research focuses on single practices (e.g., cover crops). Research from Iowa State University published this week in the Journal of Extension examines factors that influence Iowa farmers’ simultaneous use of multiple practices. The primary finding was that farmers who are more engaged in agricultural social networks tend to adopt more diverse nutrient management practices.
Farmer Social Networks
The article, “Understanding Predictors of Nutrient Management Practice Diversity in Midwestern Agriculture,” co-authored by Hanna Bates, Program Assistant at the Iowa Water Center at Iowa State University and J. Arbuckle, Iowa State University Extension Sociologist, draws on data from the 2012 Iowa Farm and Rural Life Poll. The research examined relationships between information format preferences, information sources, farm organization involvement, and opinion leadership and farmers’ use of diverse nutrient management practices. The results showed that farmers who prefer to learn about nutrient management through in-person formats such as field days, farmers who are more involved in agriculture and natural resource conservation organizations, and those who consider themselves to be opinion leaders tend to employ a more diverse range of nutrient management practices.
“The finding that farmers who prefer face-to-face learning formats for nutrient management information tend to use more diverse practices is important,” said Bates. “Given the recent increase of online webinars, publications, and social media campaigns as means to reach farmers, this result suggests that in-person formats are still valuable.”
Results also highlight the important role that agriculture and natural resource organizations can play in encouraging nutrient management practice adoption. Numerous organizations have initiated or expanded conservation programs and research projects to help farmers reduce nutrient loss.
“Farmers who are more involved in these organizations used significantly more practices,” Bates said. “This result suggests that efforts to experiment and share information about practices such as cover crops and bioreactors are paying off.”
Another key finding in the study was a positive relationship between opinion leadership and use of diverse nutrient management practices. Opinion leaders are community members whose opinions and actions can influence others. The study asked farmers to rate the degree to which they take leadership roles, are role models to other farmers, or are a source of advice for others, such as extension staff and crop advisers. Farmers who viewed themselves as opinion leaders tended to use more nutrient management practices.
“Opinion leaders can be a critical component of outreach at the local level,” said Bates. “Public recognition programs, such as the Iowa Farm Environmental Leader Award, may provide insight into who are the key players who are influencing change in rural communities.”
Future Directions for Conservation Service Providers
The findings provide positive reinforcement for efforts to engage farmers in conservation networks. On the flip side, however, the authors of the study highlight that more needs to be done to reach out to farmers who are less connected within agricultural community social networks.
“Farmers who are less involved in agricultural and conservation organizations and see themselves as less central in the ag community reported fewer nutrient management practices,” noted co-author Arbuckle. “These results point to challenges for conservation service providers because farmers who are likely in need of conservation assistance appear to be the hardest to reach. The conservation community needs to develop different strategies to engage such farmers.”
“Understanding Predictors of Nutrient Management Practice Diversity in Midwestern Agriculture” and “Iowa Farmers’ Nitrogen Management Practices and Perspectives” are available at the provided hyperlinks. More information about Sociology Extension and the Iowa Water Center can be found online.
Soil and water quality improvements in your backyard
Post written by Hanna Bates, Program Assistant at the Iowa Water Center
Urban zones are ever expanding in Iowa with new houses, apartment complexes, and businesses emerging every year. Construction in urban zones often causes negative impacts on the soil, including compaction, which can thwart root zone growth in green spaces and may lead to erosion and water quality impairments. A new study by Logsdon et al. in the Journal of Water Resource and Protection shows that compost has the ability to improve soil and water quality in post construction sites in urban areas.
Researchers examined lawn grass plots and prairie plots that had simulated construction activities, such as driving over the plots with a tractor. This activity mimics the increase of soil compaction that occurs at construction sites due to the heavy machinery used. The plots received a treatment with three types of compost application methods: compost with aeration, rototill and compost, and surface compost. These plots were compared against bluegrass, which is a traditional lawn grass, without compost. Plots then underwent a rainfall scenario with the use of a rainfall simulator. Researchers measured numerous variables in the soil including soil water, bulk density (the degree of compaction), and morphology (the observable elements of the soil).
The study found that the use of compost lessened the bulk density in the soil (Logsdon et al 2017). High bulk density is an indicator that the soil has low absorbency for water and limits plant growth. By lowering bulk density, there is an increased ability to support healthy plant life and increase the water retained in the soil. In this study, compost additions not only provided the benefit for soil health, but it also darkened the soil more than the addition of topsoil. The study also found that when compost was combined with prairie grasses, it increased infiltration and minimized runoff and sediment loss when compared to bluegrass lawn.
If you’re a developer or even a homeowner, it may be worthwhile to consider composting and planting prairie rather than traditional lawn grass. It will not only keep your soil in place, but it will make a positive impact on the surrounding environment and lessen the stress on the public water infrastructure.
Logsdon, S.D., Sauer, P.A. and Shipitalo, M.J. (2017) Compost Improves Urban Soil and Water Quality. Journal of Water Resource and Protection, 9, 345-357. 7. https://doi.org/10.4236/jwarp.2017.94023.
Hanna Bates is the Program Assistant at the Iowa Water Center. She has an MS in Sociology and Sustainable Agriculture from Iowa State University. She is currently pursuing an MBA with a leadership certificate from the University of Iowa.
Summer Update from the IWC Graduate Student Research Grant Program: Nathan Young
Post submitted by Nathan Young, a PhD student co-majoring in Geology and Environmental Science here at Iowa State University.
Over the past 30 years, computer simulations of groundwater flow have become a standard tool for investigating water quality and quantity issues across the globe. Because of a number of limitations, ranging from data availability to available computer power, these simulations (or “models”) contain a number of simplifying assumptions that prevent them from being perfect representations of the location being studied. For instance, if the subsurface was composed primarily of sand with some gravel mixed in, we may tell the model that the subsurface is only composed of sand to simplify the model and make it run faster. While these assumptions may be acceptable under most circumstances, several common assumptions made about the subsurface in Iowa may in fact impede our understanding of how water and nutrients are moving throughout the state. In Iowa’s till dominated watersheds, the subsurface is commonly treated as a fairly homogenous low-permeability material, while in reality, ultra-small-scale cracks (or fractures) present in this material provide pipe-like pathways through which water and nutrients can move very rapidly. These fractures are often omitted from models due to the massive amount of computer power required to include them in the type of watershed-scale investigations that would be conducted for the purposes of evaluating regional water quality.
In spring 2017, I was awarded funding in the Iowa Water Center Graduate Student Supplemental Research Competition for my project titled, “Simulation of Watershed-Scale Nitrate Transport in Fractured Till Using Upscaled Parameters Obtained from Till Core.” My research seeks to accomplish two goals: to develop a method to include fractures in watershed-scale models, and then to evaluate the extent to which these ultra-small-scale fractures enhance groundwater flow and nutrient transport at the watershed scale.
This past summer I have made significant progress on my project on a number of fronts. My laboratory experiments on a series of 16x16x16 cm sediment samples excavated from the Dakota Access Pipeline trenches are ongoing, but they are progressing forward. I am currently conducting flow experiments on the samples using groundwater spiked with a chemical tracer. These samples contain small-scale cracks, called fractures, which provide pathways for very rapid movement of fluid and tracer in what would otherwise be a largely impervious material. By measuring the flow rate of fluid coming out of the sample, as well as the concentration of tracer that this effluent contains, I can quantify to what degree these fractures are enhancing flow within the sample. Early results of this work show that as we move deeper in the subsurface, water moves through the samples more slowly (which is what we would expect to see) yet these flow rates are still higher than we would find if the samples did not contain fractures. Furthermore, tracer concentrations in the sample effluent indicate that the fractures are providing preferential pathways for the tracer to flow through, resulting in tracer exiting the sample much sooner than if it were unfractured. I have been fortunate to have the assistance of two undergraduates, Jay Karani ’19, and Kate Staebell ’17, in setting up these experiments and analyzing the resulting output. This work would have taken much longer without their help!
I have also been working to develop a set of new computational methods that will allow for the role that these fractures play in groundwater flow and solute transport to be included in watershed-scale computer models. Previously, accounting for groundwater flow in fractures was too computationally intensive to include in models larger than the size of a small field. Yet the early results of my work suggest that we may have found a method to circumvent this computational limitation by computing a new set of flow parameters using sophisticated, small-scale groundwater flow simulations and field data. I presented some preliminary results of this work at the 2017 MODFLOW and More conference in Golden, Colorado, this past May, and was awarded 2nd place for graduate student presentations. A short paper on this work was also published in the conference proceedings. I am currently finalizing my results in preparation for a talk I will be giving at the Geological Society of America’s National meeting in Seattle later this month. I am also in the process of writing up the results for publication, and hope to have one of two manuscripts ready for submission by the end of the semester.
Finally, I was invited to visit Laval University in Quebec City, Canada this past August to work with Dr. René Therrien, a professor in the Department of Geology and Geological Engineering who developed the groundwater model I am using in my research. With the help of Dr. Therrien and his research group, I was able to accomplish in two weeks what would have likely taken me three months on my own. I have already been invited back to work with them again in summer 2018. We are working together to write a grant proposal to secure funding for that visit. I am confident that continued work with my collaborators at Laval University will enable me to include more detail in my study area, Walnut Creek watershed, into the overall model of the watershed I am currently building.
Get to know your soil
Photos of the 2017-2018 Agronomy in the Field cohort for Central Iowa at the ISU Field Extension Education Lab. Photos by Hanna Bates.
An education in soil sampling
Last week I attended Agronomy in the Field, led by Angie Reick-Hinz, an ISU field agronomist. The workshop focused on soil sampling out in a field. The cohort learned a lot of valuable insight into not only the science of soil sampling, but also practical knowledge from out-in-the-field experiences.
Taking soil samples in a field is critical in making decisions about fertilizer, manure, and limestone application rates. Both over and under application can reduce profits, so the best decision a farmer can make is based on a representative sample that accurately shows differences across his/her fields.
What do you need?
- Sample bags
- Field map
- Soil probe
- Bucket
When do you sample?
After harvest or before spring/fall fertilization times. Sampling should not occur immediately after lime, fertilizer, or manure application or when soil is excessively wet.
Where do you sample?
Samples taken from a field should represent a soil area that is under the same type of field cultivation and nutrient management. According to ISU Extension, the “choice of sample areas is determined by the soils present, past management and productivity, and goals desired for field management practices.”* See ISU Extension resources for maps and examples for where in the field to take samples.
Most importantly…
Like with everything that happens out in the field, it is important to keep records on soil testing so that you can evaluate change over time and the efficiency of fertilizer programs. As we say at the Iowa Water Center, the more data, the better! The more we learn about the soils, the better we can protect and enhance them. Healthy soils stay in place in a field and promote better crop growth by keeping nutrients where they belong during rain events. Not only can we monitor soil from the ground with farmers, but with The Daily Erosion Project. These combined resources, with others, can provide the best guidance in growing the best crop and protecting natural resources.
Interested in Agronomy in the Field? Contact Angie Rieck-Hinz at amrieck@iastate.edu or 515-231-2830 to be placed on a contact list.
* Sawyer, John, Mallarino, Antonio, and Randy Killorn. 2004. Take a Good Soil Sample to Help Make Good Decisions. Iowa State University Extension PM 287. Link: https://crops.extension.iastate.edu/files/article/PM287.pdf

Hanna Bates is the Program Assistant at the Iowa Water Center. She has a MS in Sociology and Sustainable Agriculture from Iowa State University. She is also an alumna of the University of Iowa for her undergraduate degree.
Identifying Indicators for Soil Health
Breaking down our knowledge of soil enzymes
Post written by Marshall McDaniel, Assistant Professor in the Department of Agronomy at Iowa State University
As mentioned in a recent Washington Post article, there is a zoo beneath our feet in the soil. There are three properties of the soil, which are physical, chemical, and the biological properties. The emphasis on soil biology is, in large part, what separates soil health from the concepts of soil quality and the physical properties of soil (also known as soil tilth). After all, only something that is living can be healthy (or unhealthy). Many soil organisms are like us humans in that they require carbon as their main source of food in order to grow and reproduce. Extracellular enzymes are proteins produced by microorganisms in soil to acquire carbon and nutrients from soil organic matter.
The McDaniel Lab was one of five to receive the Soil Health Literature and Information Review Grants from the Soil Health Institute. We will do a quantitative literature review on two of these enzymes – beta-glucosidase and polyphenol oxidase. Beta-glucosidase can generally be thought of as being used for easily broken down, or labile, forms of soil carbon, and polyphenol oxidase for recalcitrant carbon. In other words, think of labile carbon as a buffet of ‘yummy and healthy’ food that is nutritious and easy-to-digest for soil microbes, while recalcitrant carbon can be thought of as the equivalent of broccoli stems to human digestion. We want to manage soils so that there is a large amount of the ‘yummy and healthy’ soil carbon for microbes to eat, and less of the ‘broccoli stems’.
Where the enzymes come in is that soil microbes will produce more of the beta-glucosidase enzyme if there is more ‘yummy and healthy’ forms of carbon in the soil, because it helps them to metabolize this form of carbon. Conversely, if all you have left in the soil are ‘broccoli stems’, then as a soil microbe you are going to produce more polyphenol oxidase to metabolize this difficult to break down source of food. Therefore, the ratio of these two enzymes holds promise as a good biological soil health indicator since it is an index of supply-and-demand for ‘yummy and healthy’ microbe food over ‘broccoli stems’.
What does this have to do with water?
Soil health and water quality go hand in hand. Improved soil health has the potential to increase water infiltration, increase water holding capacity, decrease surface runoff, decrease soil erosion, increase nutrient retention in the soil for plants, and more. By improving understanding of our soil biology, we can both better serve our natural resources and crop production.





















































































